Researchers at Beihang University have developed a novel biochar material capable of removing stable metal complexes from water with remarkable efficiency. The study, published on October 14, 2025, in the journal Biochar X, highlights the effectiveness of ferromanganese oxide-modified biochar (FMBC-600) in treating persistent metal-organic pollutants, specifically targeting copper-citrate complexes prevalent in industrial effluents.
The innovative material is coated with 100-nanometer Mn3O4 and (FeO)0.099(MnO)0.901 nanoparticles, achieving a copper removal rate of 99.5% alongside a total organic carbon reduction of 92.6%. Traditional wastewater treatment methods tend to overlook these stable metal complexes, which are often bound with organic agents like citric acid. These complexes are resistant to degradation and pose long-term ecological risks, particularly in sectors such as electroplating and textile dyeing.
Conventional techniques like precipitation and ion exchange struggle to effectively eliminate these complexes. In contrast, biochar has emerged as an inexpensive and environmentally friendly adsorbent. Despite its potential, standard biochar’s limited active sites and selectivity have undermined its effectiveness. This research addresses those challenges by designing a modified biochar with enhanced adsorption capabilities.
The team, led by Wenhong Fan, synthesized FMBC-600 through a process of impregnation and high-temperature calcination. They systematically characterized its structure and performance in removing copper-citrate complexes. Scanning Electron Microscopy (FE-SEM) revealed that the modification resulted in a rough surface uniformly coated with nanoparticles. Energy Dispersive X-ray Spectroscopy (EDS) confirmed the incorporation of iron and manganese, alongside post-adsorption copper deposition.
Fourier Transform Infrared Spectroscopy (FTIR) and X-ray Diffraction (XRD) analyses indicated a wealth of hydroxyl, aromatic, and metal-oxygen functional groups present in the modified biochar. Furthermore, X-ray Photoelectron Spectroscopy (XPS) demonstrated the involvement of iron and manganese in redox processes, contributing to adsorption through electron transfer and surface complex formation.
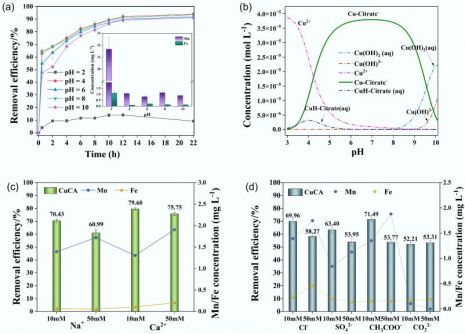
The research team optimized the adsorption process, identifying the optimal conditions as an Fe:Mn ratio of 1:4, a manganese concentration of 0.03 M, and pyrolysis at 600 °C. Under these conditions, the material achieved a rapid adsorption of copper within 30 minutes and maintained stability across a pH range of 4 to 10. Importantly, FMBC-600 demonstrated strong performance even in the presence of competing ions such as sodium, calcium, chloride, and sulfate, indicating its selectivity and resilience.
Kinetic modeling showed that the adsorption process aligns with a pseudo-second-order equation, suggesting that chemisorption is the dominant mechanism. The Freundlich isotherm model described heterogeneous multilayer adsorption, which improves at elevated temperatures. Regeneration tests confirmed that FMBC-600 retained approximately 80% of its efficiency after two cycles, underscoring its reusability.
Overall, FMBC-600 presents an advancement in sustainable water treatment, showcasing excellent stability, reusability, and adsorption efficiency. Its straightforward, low-cost production process positions it well for large-scale applications in managing industrial effluents, particularly in sectors like electroplating and chemical manufacturing. Moreover, this technology has potential for broader applications, including soil remediation, where it can help mitigate heavy metal accumulation in agricultural lands.
The integration of ferromanganese oxide-modified biochar into wastewater treatment frameworks could significantly reduce metal contamination, thereby contributing to global clean water initiatives and sustainability goals.
This research was supported by the National Natural Science Foundation of China and other regional funding sources, emphasizing the collaborative efforts driving innovation in environmental science.
For more detailed insights, the full study is accessible via the DOI: 10.48130/bchax-0025-0001.